
This is a promotional website intended for UK healthcare professionals.
Colobreathe® (colistimethate sodium) Prescribing Information can be found here.
Adverse Event Reporting Information can be found at the end of this page.

This is a promotional website intended for UK healthcare professionals.
Colobreathe® (colistimethate sodium) Prescribing Information can be found here.
Adverse Event Reporting Information can be found at the end of this page.
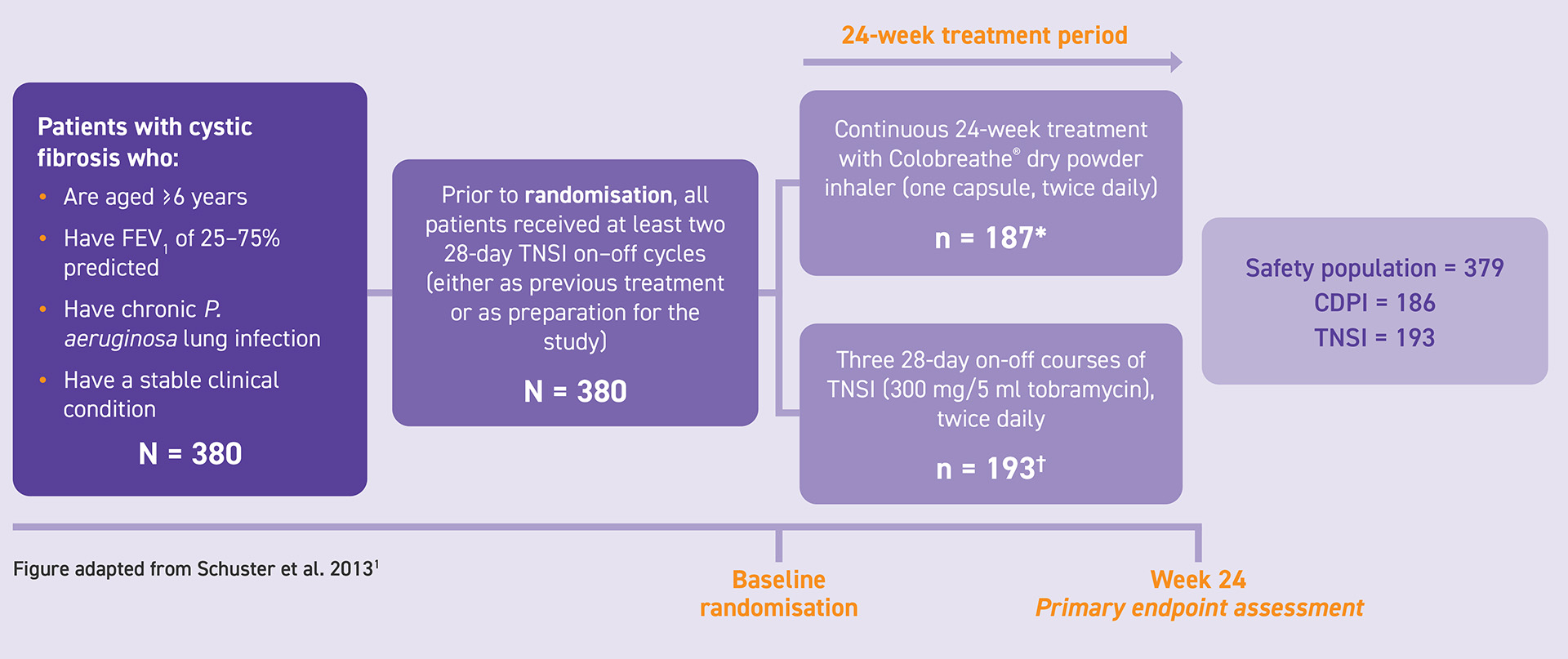
Patients were randomly assigned to receive continuous treatment over a 24-week period with Colobreathe® DPI (one capsule of 1,662,500 IU twice daily) or to three 28-day, on-off courses of tobramycin nebuliser solution for inhalation (TNSI; 300 mg/5ml twice daily) using a PARI LC Plus nebuliser. Prior to randomisation, all patients received at least two 28-day TNSI on-off cycles.
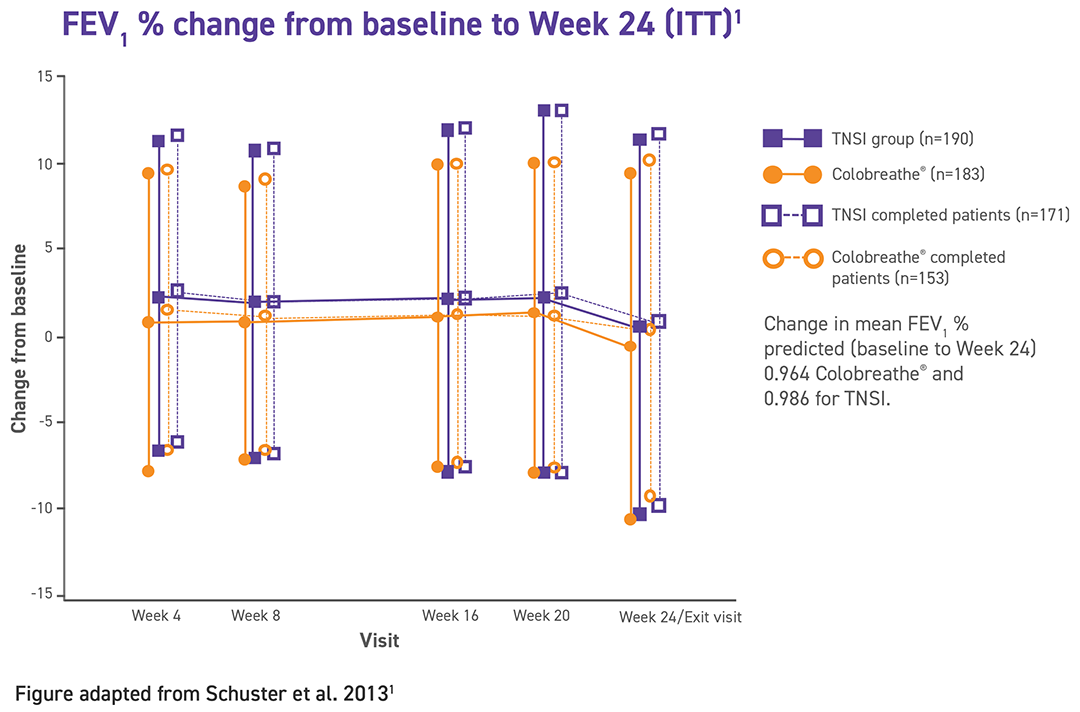
The primary endpoint was the change in mean FEV1 predicted from baseline at week 24.
Primary endpoint:
Change in mean FEV1 predicted from baseline at week 24.
Secondary endpoints:
Antibiotic susceptibility of Pseudomonas aeruginosa to colistin and tobramycin; Quality of Life CFQ-R; change in FVC, absolute FEV1 and FEF25-75; compliance to study medication; adverse events; patient view of trial treatment including time to administer, ease of use and convenience; patient preference.
| Colobreathe®(n=186)* | TNSI(n=193) | Total(n=379) | |
|---|---|---|---|
| Total number of adverse events | 1232 | 1194 | 2426 |
| Cough | 193 (15.7) | 123 (10.3) | 316 (13.0) |
| Dysgeusia | 132 (10.7) | 62 (5.2) | 194 (8.0) |
| Dyspnoea | 81 (6.6) | 98 (8.2) | 179 (7.4) |
| Lower respiratory tract infection | 79 (6.4) | 85 (7.1) | 164 (6.8) |
| Throat irritation | 94 (7.6) | 63 (5.3) | 157 (6.5) |
| Productive cough | 62 (5.0) | 76 (6.4) | 138 (5.7) |
*One patient was randomised but received no treatment.1
Table adapted from Schuster et al 20131
| System Organ Class | Very Common (≥ 1/10) | Common (≥ 1/100 to <1/10) |
|---|---|---|
| Nervous system disorders | Balance disorder, headache | |
| Ear and labyrinth disorders | Tinnitus | |
| Respiratory, thoracic and mediastinal disorders | Dyspnoea, cough, dysphonia, throat irritation | Haemoptysis, bronchospasm, asthma, wheezing, chest discomfort, lower respiratory tract infection, productive cough, crackles lung |
| Gastrointestinal disorders | Dysgeusia | Vomiting, nausea |
| Musculoskeletal and connective tissue disorders | Arthralgia | |
| General disorders and administration site conditions | Pyrexia, asthenia, fatigue | |
| Investigations | Forced expiratory volume decreased |
For full list of adverse events consult the Summary of Product Characteristics.
*The safety of Colobreathe® was assessed in a 24-week clinical study in a total of 237 subjects (225 CF patients and 12 healthy volunteers). In the 24-week clinical study, where Colobreathe® was administered twice daily to adults and children aged 6-17, the adverse reactions identified in the paediatric population were similar to that for the overall population. The most commonly reported adverse reactions as a percent of Colobreathe® treated paediatric patients were: cough (55%), unpleasant taste (51%), throat irritation (34%), dyspnoea (10%) and dysphonia (10%).2
| Contraindications |
|---|
| Colobreathe® should not be used in patients with hypersensitivity to the active substance, colistin sulphate or polymyxin B. |
| Special warnings and precautions | |
|---|---|
| Bronchospasm and coughing | Bronchospasm or coughing may occur on inhalation. These reactions usually disappear or significantly diminish with continued use and may be ameliorated by appropriate treatment with beta2-agonists prior to or following dry powder colistimethate sodium inhalation. If bronchospasm or coughing remain problematic, withdrawal of treatment should be considered. |
| Haemoptysis | Haemoptysis is a complication in cystic fibrosis and is more frequent in adults. The use of colistimethate sodium in patients with clinically significant haemoptysis should be undertaken or continued only if the benefits of treatment are considered to outweigh the risks of inducing further haemorrhage. |
| Acute respiratory exacerbation | If acute respiratory exacerbations develop additional intravenous or oral antibacterial agent therapy should be considered. |
| Oral fungal super-infection | After each inhalation of Colobreathe®, the mouth should be rinsed with water. The rinse should not be swallowed. Rinsing may reduce the risk of developing oral fungal super-infection during treatment and may also reduce the unpleasant taste associated with colistimethate sodium. |
| Nephrotoxicity/ neurotoxicity | There is very low transpulmonary absorption of colistimethate after inhalation of Colobreathe®. Care should still be taken in administering Colobreathe® to patients who are known to have a propensity for nephrotoxic or neurotoxic events. Caution should be taken with concomitant use of Colobreathe® and parenteral or nebulised colistimethate sodium. Caution should be taken with concomitant use of colistimethate sodium and potential nephrotoxic or neurotoxic medicinal products, including nondepolarising muscle relaxants. |
| Other | Colobreathe® should be used with extreme caution in patients with myasthenia gravis because of potential for drug induced neuromuscular blockade. Colistimethate sodium should be used with extreme caution in patients with porphyria. Safety and efficacy have been assessed in controlled studies for up to 24 weeks. This medicinal product contains less than 1 mmol sodium (23 mg) per capsule, that is to say essentially ‘sodium-free’. |
For full information on contraindications, warnings and precautions please consult the Summary of Product Characteristics.
AEs, adverse events; CDPI, Colobreathe® dry powder inhaler; CF, cystic fibrosis; CFQ-R, Cystic fibrosis questionnaire- Revised; CI, confidence interval; DPI, dry powder inhaler; FEF25-75, change in forced expiratory flow between 25% and 75% of the vital capacity; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ITT, intention to treat; ITT LOCF, intention-to-treat last observation carried forward; IU, international unit; LRTI, lower respiratory tract infection; SAEs, serious adverse events; TNSI, tobramycin nebuliser solution for inhalation.
References:
1. Schuster A, Haliburn C, Döring G, et al. Thorax. 2013;68:344–350.
2. Colobreathe® Summary of Product Characteristics. Available here.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk or search for the MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Essential Pharma on +44 (0) 1423 850700 or at EssentialpharmaUK@EU.ProPharmaGroup.com.
Essential Pharma Ltd, 8A Crabtree Road, Egham, Surrey, TW20 8RN, United Kingdom
Colobreathe® is a registered trademark of Essential Pharma. Copyright © 2026 Essential Pharma
MAT-COL-GB-0004-1224 May 2025
